Recent studies have shown the therapeutic benefits of cannabidiol (CBD), which is a non-intoxicating component of Cannabis sativa. Previously, the association of Δ9-tetrahydrocannabinol (Δ9-THC) with CBD limited studies on its medicinal benefits.
The Agricultural Improvement Act of 2018 (Agricultural Act 2018) legalized the use of products from parts of the sativa cannabis plant, also known as hemp. This led to the rapid growth of the CBD industry.

To study: Label accuracy of unregulated cannabidiol (CBD) products: Measured concentration compared to label statement. Image credit: IRA_EVVA / Shutterstock.com
Fund
In June 2018, a purified oral CBD solution known as Epidolex® received approval from the U.S. Food and Drug Administration (FDA) for the treatment of three forms of epilepsy.
However, with the exception of Epidolex®, other CBD products remain unregulated in the U.S. In June 2019, the FDA held a general public hearing to hear scientists’ concerns about CBD regulation.
Many research groups, along with the FDA, have identified numerous label accuracy issues for various CBD products. This problem was not limited to the United States, as similar reports have been published in the Netherlands, the United Kingdom, and Italy.
A new study in the Journal of Cannabis Research sought to determine the CBD content of various products and compare it to label claims. The products evaluated in this study were purchased from several Kentucky Central stores, as well as online retailers, between April 2, 2021 and May 9, 2021.
About the study
The current study included a total of 80 samples, 44 of which were purchased from online retailers based in the United States. The remaining 36 samples were purchased from local retailers in central Kentucky.
Altogether, the samples included in this study were produced by 51 different brands. Epidiolex® was also used as a positive control. All CBD products were tested immediately upon opening and before their expiration date.
To determine the accuracy of the label, ± 10% of the allowed variance was used. Products with a CBD concentration above 110% of the labeled value were considered sub-labeled, while those with less than 90% of their labeled CBD content were considered over-labeled. Products with a CBD concentration between 90 and 110% of the labeled CBD content were considered to be accurately labeled.
All samples were then prepared and analyzed by liquid chromatography-tandem mass spectrometry (LC-MS / MS).
Study results
Of the 80 samples tested, 31% were sub-labeled, 54% were accurately labeled, and 15% were over-labeled. Of the 44 products purchased online, 25% were under-labeled, 61% were accurately labeled, and 14% were over-labeled. Of the 36 products purchased locally, 39% were under-labeled, 17% over-labeled and 44% accurately labeled.
CBD concentrations ranged from 2.9 to 61.3 mg / ml for all unregulated samples, with labeled CBD concentrations ranging from 17 to 159%. The average amount of CBD for low-labeled products was 121% of the label statement, compared to 61% of over-labeled products.
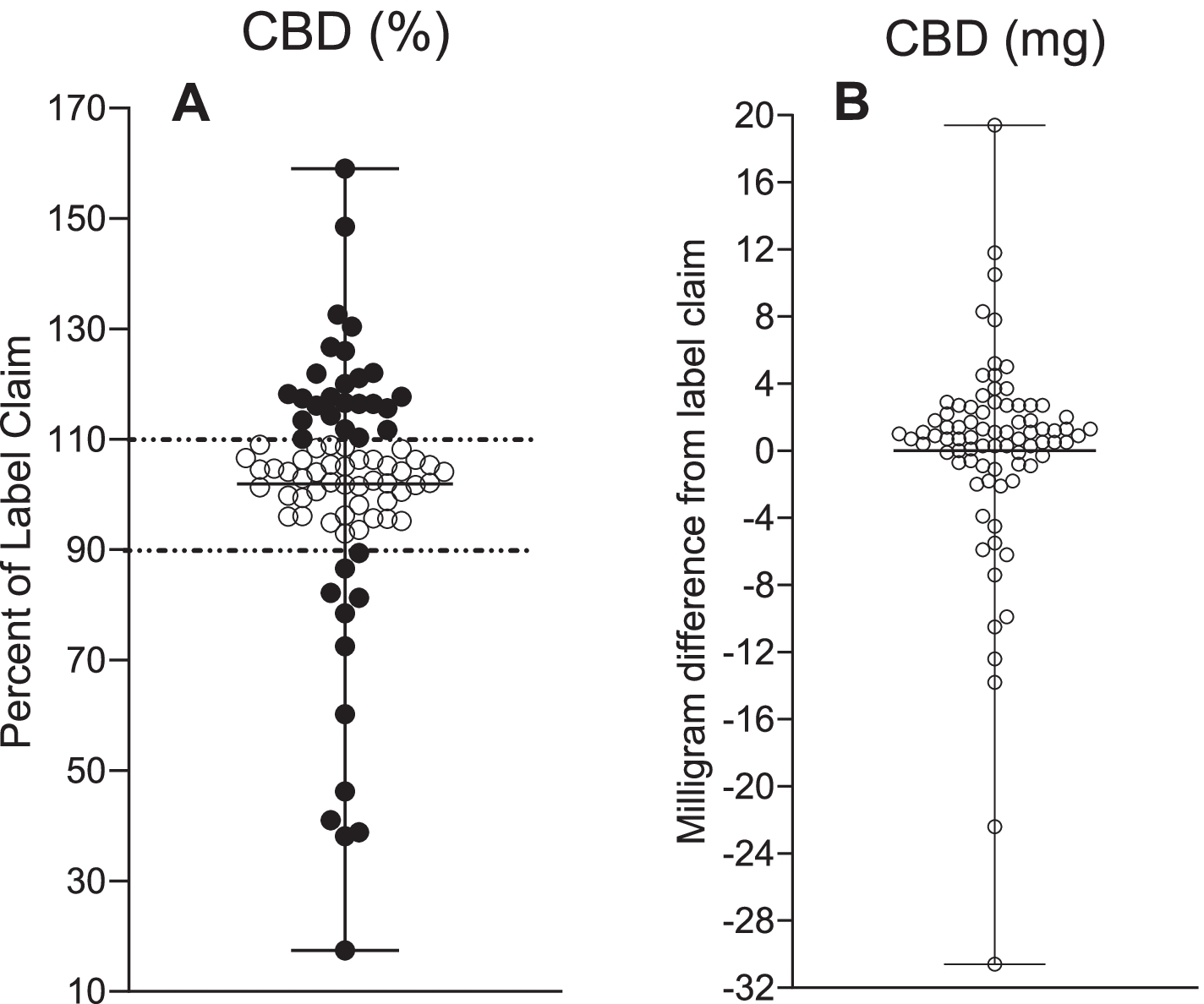 CBD measurements in 80 commercially available CBD oil products and Epidiolex®. A The percentage of CBD label assertion content with a tolerance of ± 10% indicating lower labeling (> 110%) and excessive labeling (<90%). B Deviation of the CBD label statement in milligrams
CBD measurements in 80 commercially available CBD oil products and Epidiolex®. A The percentage of CBD label assertion content with a tolerance of ± 10% indicating lower labeling (> 110%) and excessive labeling (<90%). B Deviation of the CBD label statement in milligrams
Conclusions
The current study provides evidence that CBD products from several countries around the world often had CBD concentrations inconsistent with the label statements. Such inaccurate labeling may pose a risk to the safety of consumers using CBD products for medical treatment.
Therefore, clear regulations from both federal and state agencies, as well as good manufacturing practices, are required to ensure that CBD product label statements are accurate.
Limitations
The study has certain limitations. First, the study included only hemp-derived products. Second, only CBD concentrations were reported, while other cannabinoid concentrations were not reported. The current study also did not involve a formal sampling protocol.
Magazine reference:
- Johnson, E., Kilgore, M. and Babalonis, S. (2022). Label accuracy of unregulated cannabidiol (CBD) products: Measured concentration compared to label statement. Journal of Cannabis Research. doi: 10.1186 / s42238-022-00140-1.










